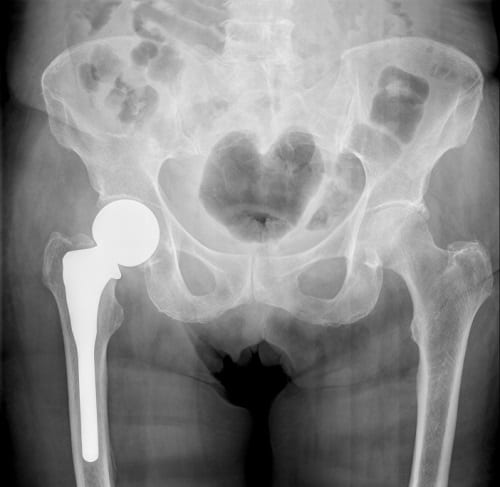
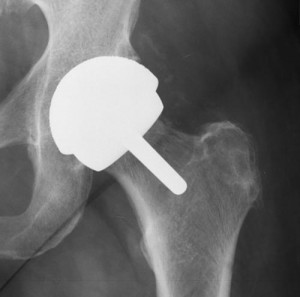
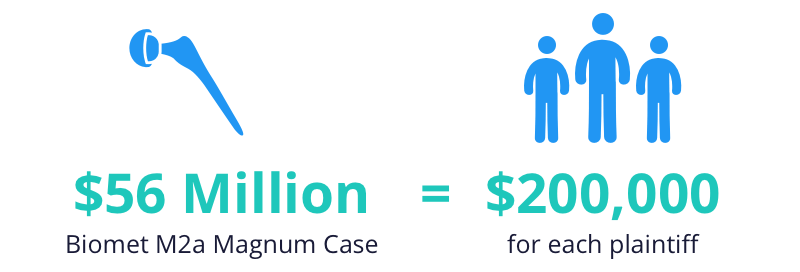Biomet Hip Lawsuit Update
Biomet mom hip replacements other than the m2a 38 and the m2a magnum such as the m2a taper ringloc or a recap will receive 20 000 without regard to any qualifying or discounting criteria.
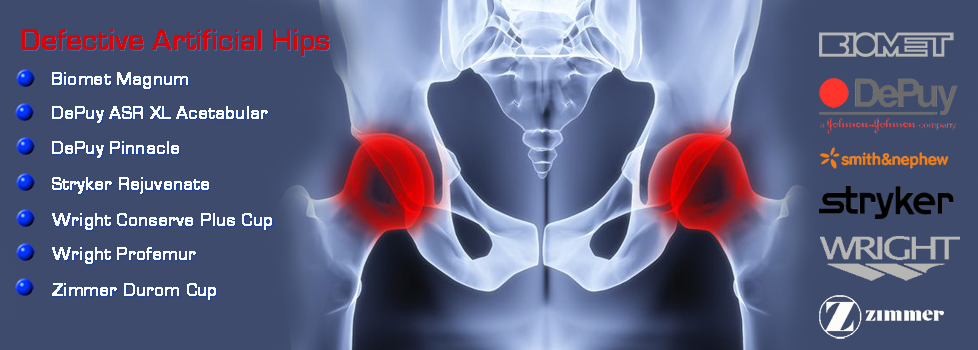
Biomet hip lawsuit update. Despite the fact that there has been no recall lawsuits are being filed against biomet for their failure to ensure the safety of their products and their patients. Biomet magnum lawsuit. In total biomet has shelled out 56 million in injury claims involving the m2a magnum implant. Lawyers represent people harmed by biomet m2a magnum hip replacement.
The biomet m2a 38 and the m2a magnum are metal on metal hip implants with a history of serious complications. Biomet kept selling the magnum hip in the united states until 2014 even after worldwide recalls four years earlier for very similar hips implants. Out of this settlement plaintiffs received a base rate of 200 000 apiece though some adjustment to that figure could be made depending on various factors such as whether the individual had received resurfacing surgery or if the case was filed after the. Thousands of lawsuits have already been filed and billions of dollars won against negligent hip implant manufacturers like zimmer biomet.
On october 4 2013 a class action lawsuit was filed in canada against the manufacturers and distributors of biomet hip implants. Anatomic individuality or extremely difficult reconstructive cases may require the development of a patient specific hip implant. Most people who sued biomet in a previous class action lawsuit received 200 000 each to settle. Claimants who received biomet metal on polyethylene devices or metal on metal hip replacements other than the m2a 38 and the m2a magnum m2a taper ringloc or recap received 20 000.
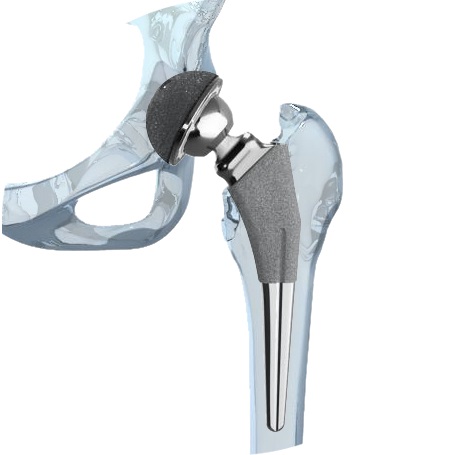
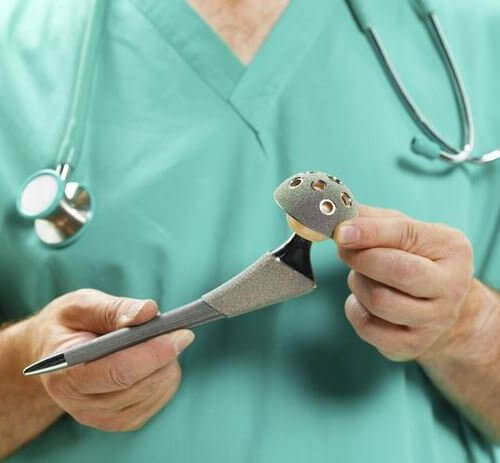
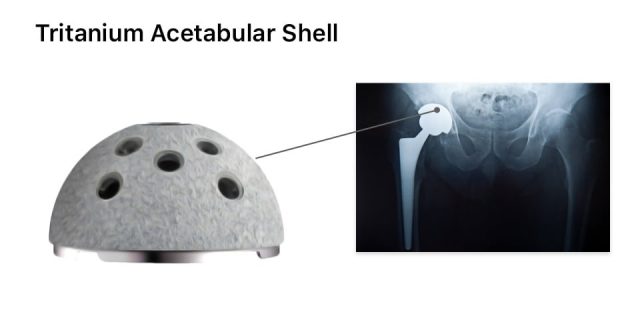
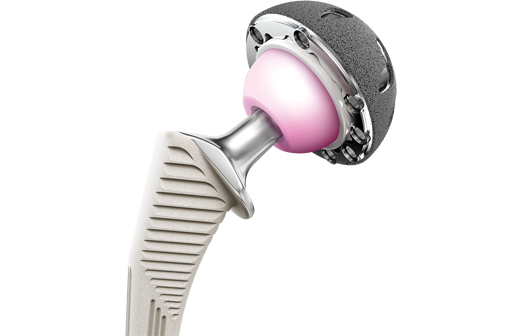
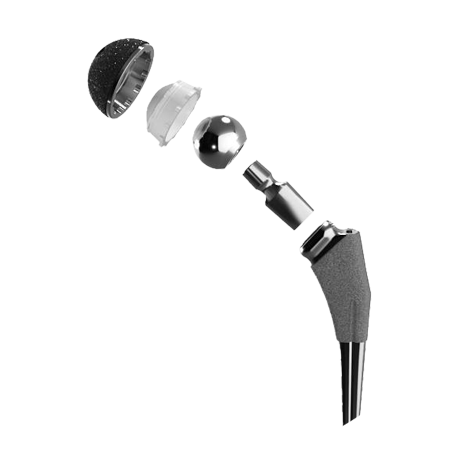
The biomet m2a magnum hip device is a metal on metal hip replacement system consisting of a metal cup and a modular head. 6 plaintiffs who received any type of biomet mom hip for the first time as part of a. The lawsuit alleges that the defendants were negligent in the research design manufacture regulatory licensing sale and post market monitoring of the biomet implants. Cases barred by statutes of limitation received 20 000.
Biomet continues to market its hip replacement devices as safe and effective and more so than other hip replacement systems. In such cases zimmer biomet s patient matched implant pmi department is available to work directly with surgeons to address the most severe cases of bone loss or deformity using ct x ray or mri data the pmi team can provide a solution for even the most. Cases involving a hip replacement system implanted after january 27 2012 received 20 000. Patients across the united states have filed lawsuits demanding biomet take responsibility for making and selling defective hips.
Strangely biomet continues to use retton s image and story to promote the success of the magnum hip.